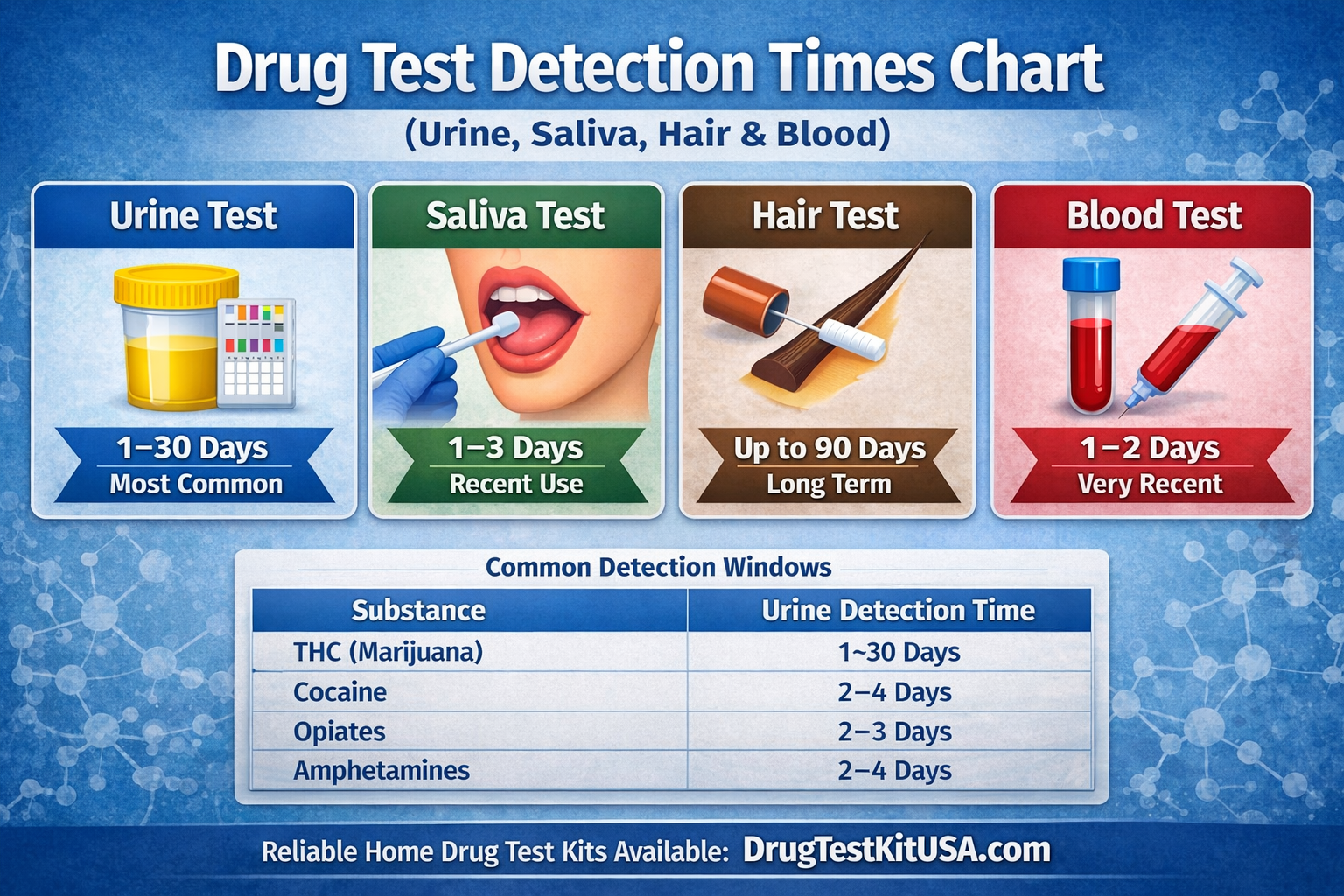
Drug Test Detection Times Chart (Urine, Saliva, Hair & Blood)
Drug test detection times vary depending on the substance, the type of test used, and individual biological factors. Understanding how long drugs remain detectable is

Game-Changer: FDA Grants Clearance for First COVID-19 Home Antigen Test
Date: November 09, 2023
Today, the U.S. Food and Drug Administration cleared for marketing the first over-the-counter (OTC) antigen test for COVID-19. ACON Laboratories’ Flowflex COVID-19 Antigen Home Test, originally authorized for emergency use in 2021, is now the second home COVID-19 test to successfully complete a traditional FDA premarket review pathway, and the first indicated for use in children under 18. Today’s announcement follows clearance of a molecular home test earlier this year.
“This marks the latest step forward in our efforts to help test developers provide Americans with continued options for safe and effective COVID tests that can be performed entirely at home,” said Jeff Shuren, M.D., J.D., director of the FDA’s Center for Devices and Radiological Health. “The FDA continues to proactively work with test developers that desire to market their products beyond emergency use authorities. This is part of the FDA’s broader effort to advance the development and availability of at-home tests for a variety of medical conditions to expand patient access to testing.”
The Flowflex COVID-19 Antigen Home Test is a visually-read test cleared for OTC home use by symptomatic individuals within six days of symptom onset. It is cleared for individuals aged 14 years or older testing themselves, or adults testing individuals aged two years or older. In a study reviewed by the FDA, this test correctly identified 89.8% of positive and 99.3% of negative samples in individuals with signs and symptoms of upper respiratory infection.
As with antigen tests authorized for emergency use, this test is intended to be used at least twice over three days with at least 48 hours between tests. This means that a symptomatic individual with an initial negative test result should be re-tested once between 48 and 72 hours after the first test using an antigen test for COVID-19 or followed up with a molecular COVID-19 test.
The FDA reviewed the ACON Flowflex COVID-19 Antigen Home Test through the 510(k) premarket review pathway. A 510(k) is a premarket submission made to the FDA to demonstrate that a new device is substantially equivalent to a legally marketed predicate device.
###
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Source: U.S. Food & Drug Administration Press Announcement
DrugTestKitUSA is an online supplier of medical tests as well as drug & alcohol tests, supplies, and devices. The Flowflex COVID-19 Antigen Home Test is available in 1–pack and 2-pack options.

Drug test detection times vary depending on the substance, the type of test used, and individual biological factors. Understanding how long drugs remain detectable is

Drug use in the workplace is a real issue that many small to medium business (SMB) owners face. According to National Survey on Drug Use

Security at Stripe: Learn how Stripe handles security.
A PCI-certified auditor has audited Stripe. We’re a certified PCI Service Provider Level 1. This is the most stringent level of certification available in the payments industry. To accomplish this, we use the best-in-class security tools and practices to maintain a high level of security at Stripe.
Stripe forces HTTPS for all services using TLS (SSL), including our public website and the Dashboard to ensure secure connections:
We regularly audit the details of our implementation, including the certificates we serve, the certificate authorities we use, and the ciphers we support. We use HSTS to ensure that browsers interact with Stripe only over HTTPS. Stripe is also on the HSTS preloaded lists for both Google Chrome and Mozilla Firefox.
All card numbers are encrypted at rest with AES-256. Decryption keys are stored on separate machines. None of Stripe’s internal servers and daemons can obtain plain text card numbers but can request that cards are sent to a service provider on a static allowlist. Stripe’s infrastructure for storing, decrypting, and transmitting card numbers runs in a separate hosting environment, and doesn’t share any credentials with Stripe’s primary services including our API and website.
Why use PayPal?
Your information is secured.
When you pay with PayPal, your financial information is never shared with the seller, and you can pay using only your email address and password.
Security around the clock.
PayPal monitors every transaction 24/7 to help prevent against fraud, email phishing and identity theft. Every transaction is heavily guarded behind their next-level encryption.
If something seems suspicious, their dedicated team of security specialists is immediately on it to help protect you from fraudulent transactions. And remember, PayPal will never ask for any sensitive information from you in an email.
You can be sure that your transaction is protected and secure when you use PayPal at the DrugTestKitUSA checkout.