
Drug Testing at Work: Why It Matters for Small to Medium Business Owners
Drug use in the workplace is a real issue that many small to medium business (SMB) owners face. According to National Survey on Drug Use

FDA Approves First Test to Help Identify Elevated Risk of Opioid Use Disorder
DrugTestKitUSA is pleased to announce the FDA approval of the AvertD test, developed by AutoGenomics Inc., as the first-ever tool designed to assist in identifying individuals with an elevated risk for developing Opioid Use Disorder (OUD). This groundbreaking development marks a significant advancement in preemptive healthcare and addiction management.
On Dec 19, 2023, the FDA announced the approval of the first test to help identify elevated risk of developing opioid use disorder.
“The opioid crisis, one of the most profound public health issues facing the United States, calls for innovative measures to prevent, diagnose and treat opioid use disorder, including to assess the risk of developing the disorder,” Dr. Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, said in the statement announcing the approval.
The AvertD is a prescription-only genetic test for individuals aged 18 and older, intended for use with patients who have never used oral opioid painkillers and who consent to the test. Administered by a healthcare provider, the test involves collecting a DNA sample from the patient via a cheek swab. This sample is then analyzed to identify any genetic variations that could indicate an increased risk of developing opioid use disorder (OUD)..
The test utilizes innovative genetic testing technology to assess an individual’s genetic predisposition to OUD. By analyzing specific genetic markers, healthcare professionals can now identify patients who may be at higher risk of developing an addiction to opioids before they are prescribed these medications.
In addition to reducing the risk of addiction, the test also plays a role in promoting better long-term health outcomes. By identifying at-risk individuals early, healthcare providers can provide targeted interventions, such as counseling or non-opioid pain management options, to mitigate the risk of dependency. This preventative approach helps ensure that individuals are supported before they reach a point of substance abuse, allowing for healthier pain management practices and reducing the overall reliance on opioids.
This FDA approval represents a major milestone in the field of addiction medicine. By identifying individuals with a genetic predisposition to OUD, healthcare providers can tailor pain management strategies and implement alternative therapies to minimize the use of opioids post-surgery. The FDA, however, has stressed proper training, accurate labeling, and strict procedures to conduct the test because of the risk of false positive or false negative results.

In healthcare settings, opioid misuse and addiction are growing concerns. Medical professionals must ensure that patients are not abusing opioid medications, and DrugTestKitUSA provides reliable testing tools to help with this task. Our drug tests are commonly used in pain management clinics, addiction treatment centers, and other healthcare facilities to detect the presence of opioids in patients. By identifying opioid use, healthcare providers can adjust pain management strategies, offer appropriate treatment plans, and intervene early to prevent addiction.
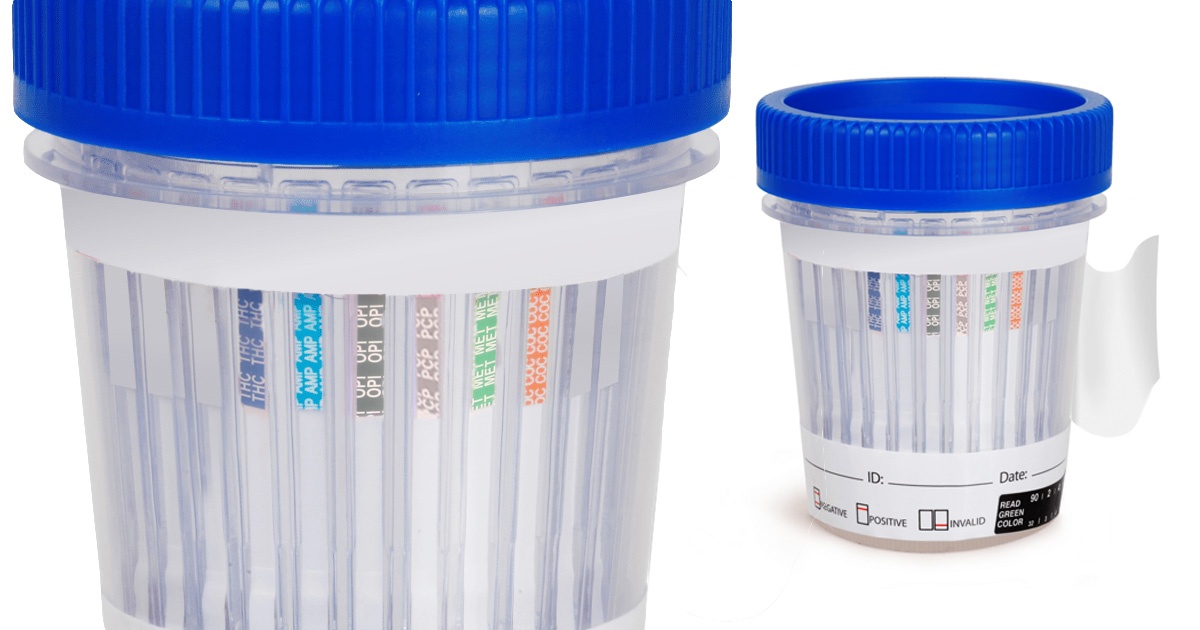
DrugTestKitUSA offers a range of reliable and easy-to-use testing solutions designed to help employers, healthcare providers, and individuals identify opioid misuse and prevent addiction. Most of our drug test kits are designed to detect a variety of opioids, including natural opiates like morphine and codeine, semi-synthetic opioids like heroin, oxycodone, buprenorphine, and hydrocodone, as well as fully synthetic opioids like methadone, tramadol, and fentanyl. These tests are essential tools in maintaining safety, well-being, and productivity in the workplace, especially in industries where safety-sensitive tasks are performed.
With the rise in opioid abuse and addiction, opioid testing is a vital part of substance abuse prevention programs. Early detection allows for immediate intervention, whether in a medical setting or at the workplace. Opioid testing can help reduce the risks associated with addiction, ensuring that individuals receive the proper treatment or support needed to recover. DrugTestKitUSA’s opioid testing solutions are a critical tool in this effort, providing peace of mind for employers, healthcare providers, and individuals alike. Filter by opiates to see all available drug tests with that configuration.

Drug use in the workplace is a real issue that many small to medium business (SMB) owners face. According to National Survey on Drug Use

The AvertD test is a prescription-only genetic test for those 18+ to assess the risk of opioid use disorder using a cheek swab.

Security at Stripe: Learn how Stripe handles security.
A PCI-certified auditor has audited Stripe. We’re a certified PCI Service Provider Level 1. This is the most stringent level of certification available in the payments industry. To accomplish this, we use the best-in-class security tools and practices to maintain a high level of security at Stripe.
Stripe forces HTTPS for all services using TLS (SSL), including our public website and the Dashboard to ensure secure connections:
We regularly audit the details of our implementation, including the certificates we serve, the certificate authorities we use, and the ciphers we support. We use HSTS to ensure that browsers interact with Stripe only over HTTPS. Stripe is also on the HSTS preloaded lists for both Google Chrome and Mozilla Firefox.
All card numbers are encrypted at rest with AES-256. Decryption keys are stored on separate machines. None of Stripe’s internal servers and daemons can obtain plain text card numbers but can request that cards are sent to a service provider on a static allowlist. Stripe’s infrastructure for storing, decrypting, and transmitting card numbers runs in a separate hosting environment, and doesn’t share any credentials with Stripe’s primary services including our API and website.
Why use PayPal?
Your information is secured.
When you pay with PayPal, your financial information is never shared with the seller, and you can pay using only your email address and password.
Security around the clock.
PayPal monitors every transaction 24/7 to help prevent against fraud, email phishing and identity theft. Every transaction is heavily guarded behind their next-level encryption.
If something seems suspicious, their dedicated team of security specialists is immediately on it to help protect you from fraudulent transactions. And remember, PayPal will never ask for any sensitive information from you in an email.
You can be sure that your transaction is protected and secure when you use PayPal at the DrugTestKitUSA checkout.