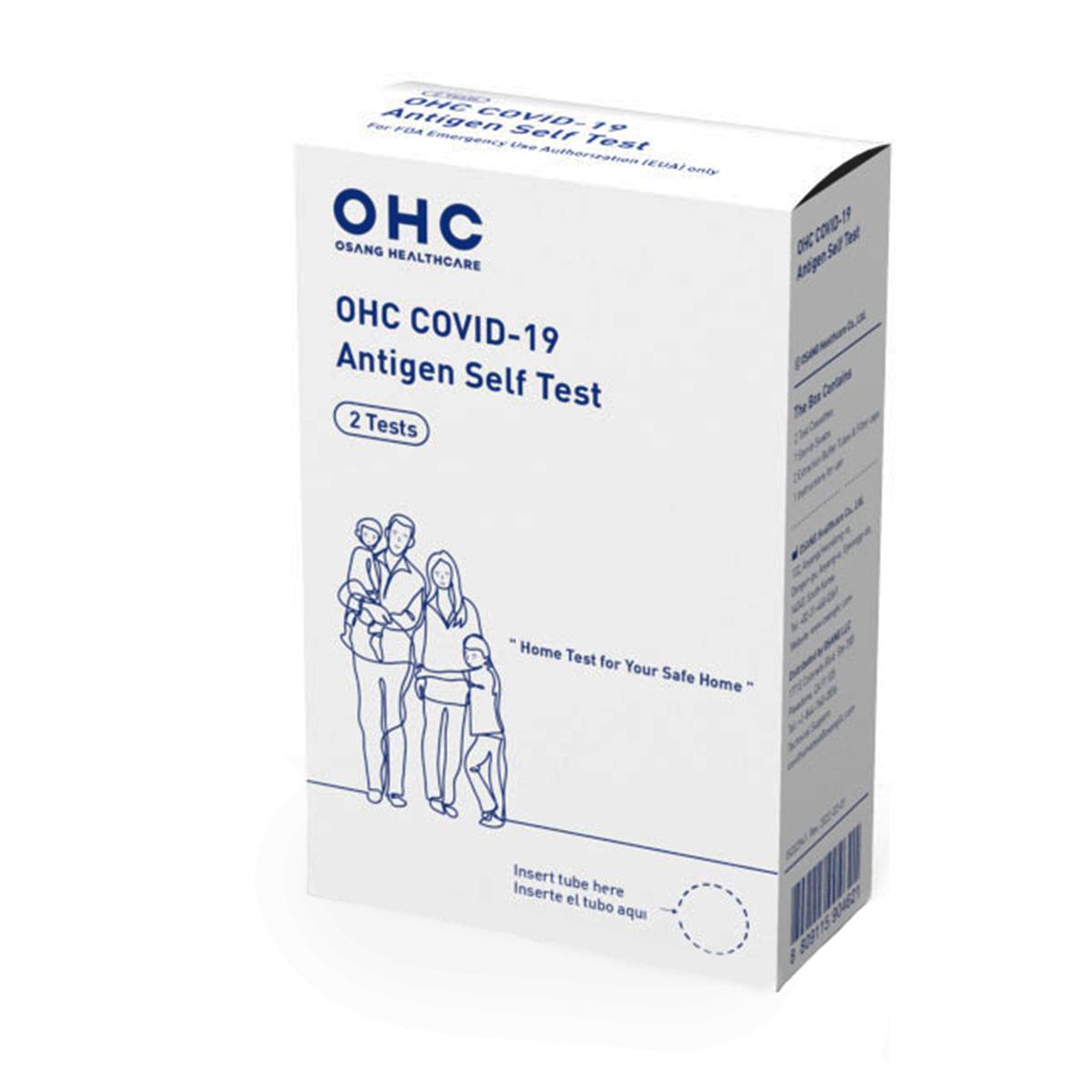
OHC COVID-19 Antigen Self Test (2 Tests/Box)
$15.00 Original price was: $15.00.$6.50Current price is: $6.50.
Price per unit: $7.50 $3.25

Product Description
The OHC COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in anterior nasal (nares) swabs from individuals when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
CALL FOR BULK PRICING
866-205-9215
Description
The OHC COVID-19 Antigen Self Test is intended for non-prescription self-use and/or as applicable, for an adult lay user testing another aged 2 years or older. The OHC COVID-19 Antigen Self Test is only for use under the Food and Drug Administration’s Emergency Use Authorization.
The “OHC COVID-19 Antigen Self Test” by OSANG Healthcare is designed for individuals with symptoms of COVID-19 within the first 7 days of symptom onset, or individuals without symptoms or other epidemiological reasons to suspect COVID-19. Those who are suspected infectious COVID-19 should perform the test at least twice over three days using “OHC COVID-19 Antigen Self Test” to detect the SARS CoV-2 antigen as quickly as possible.
The OHC COVID-19 Antigen Self Test, a high quality test you can trust.
91% relative sensitivity and 99% relative specificity✱
Prevent your loved ones from exposure to the virus with the accuracy of the OHC COVID-19 Antigen Self Test.
OHC continues to conduct studies for SARS-CoV-2 as new variants continue to emerge so that we can provide high-quality testing for you and your family.
Using the easy nasal swab test, you can self-collect samples with highly accurate and reliable results you can expect in just 15 minutes.
Children starting at the age of 14 can self test. Children from ages 2 to 13 require the help of an adult.
Feel safe and confident whether or not you have symptoms of COVID-19.
The OHC COVID-19 Antigen Self Test detects both Omicron and Delta variants.
Features and Benefits
- Detects both Omicron and Delta variants
- Easy and Affordable
- Highly Accurate Nasal Swab Test
- Fast Results in 15 minutes
- Safe for children as young as 2 years old
- For use with and without symptoms
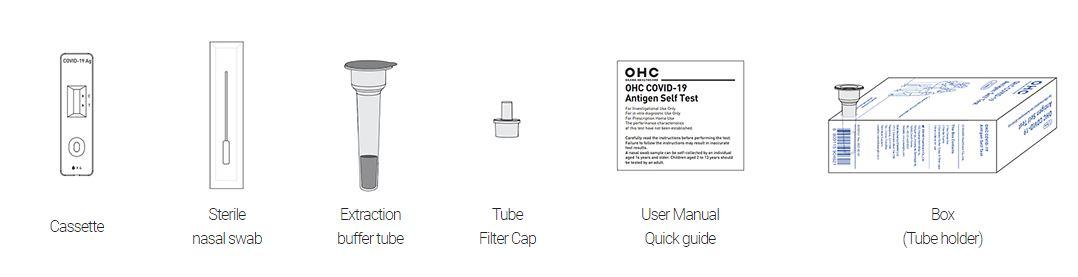
OHC COVID-19 Antigen Self Test Kit Contents:
✱Clinical data shows, OHC COVID-19 Antigen Self Test demonstrated a relative sensitivity of 91% ( 95% CI: 82.8 to 95.6%) at high viral loads (less than a cycle threshold (Ct)-value of 30) and relative specificity of 99% (95% CI: 95.2 to 99.6%). Clinical data analyzed using the minimum recommended number of low positives demonstrated that the test correctly identified 82.9% of positive samples and correctly identified 98.6% of negative samples. For more detailed information on test performance please see Section 13 of the Health Care Provider Instructions for Use. It is not possible to determine how much virus is in your sample prior to testing and a negative test does not rule out COVID-19 infection. A negative test also does not mean that you are not infectious. You can still have COVID-19 with a negative result from this test and infect other people. For this reason, it is recommended that you test at least twice (serial testing) when using this test. You should contact your healthcare provider to determine if additional testing with a highly sensitive COVID-19 molecular test is recommended. The performance of this test is still being studied in patients without signs and symptoms of respiratory infection and for serial screening. Performance may differ in these populations.
• This product has not been FDA cleared or approved but has been authorized by FDA under an EUA.
• This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
• The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of IVDs for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.
More information about COVID-19 is available at the CDC-Centers for Disease Control and Prevention.
ref: FBE00608
SKU: DTK-951500
Additional information
| Weight | 0.5 lbs |
|---|---|
| Dimensions | 5 × 2 × 1 in |