
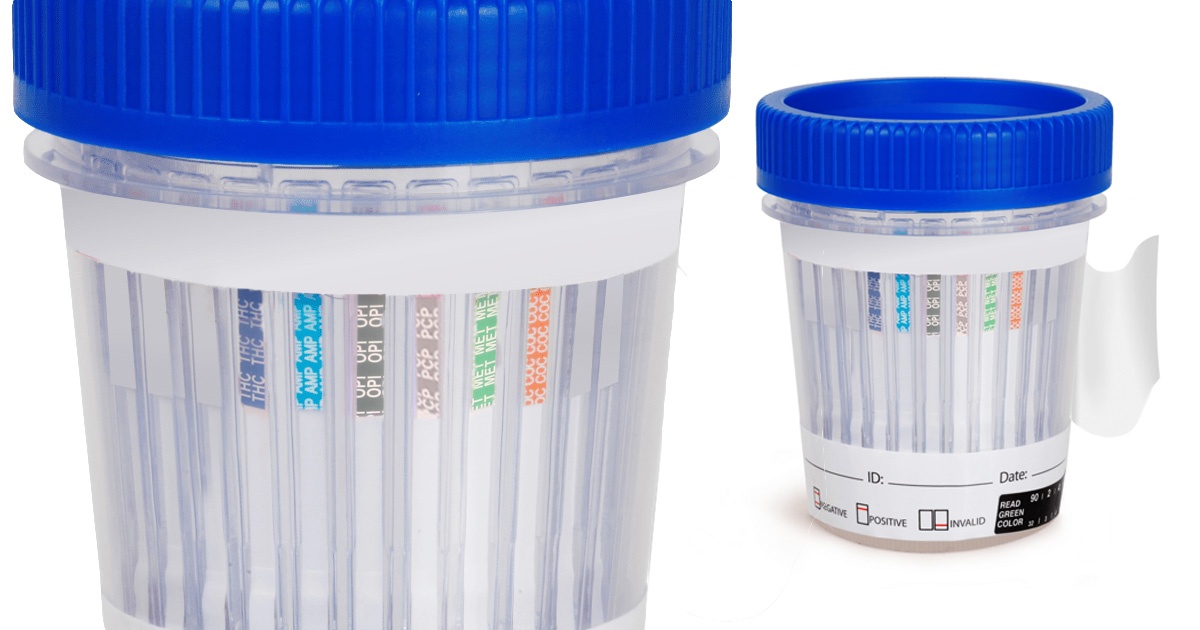
Drug Testing at Work: Why It Matters for Small to Medium Business Owners
Drug use in the workplace is a real issue that many small to medium business (SMB) owners face. According to National Survey on Drug Use

Emergency Use Authorization for Flowflex Plus COVID-19 & Flu A/B Combo Test
The Acon Laboratories Flowflex Plus COVID-19 and Flu A/B Home Test has received Emergency Use Authorization (EUA) from the FDA. This advanced diagnostic tool is designed to detect both SARS-CoV-2, the virus responsible for COVID-19, as well as influenza A and B viruses, using a single sample.
This dual-detection capability addresses a critical need, especially as flu season approaches and the COVID-19 pandemic continues to impact communities worldwide. By allowing for the simultaneous detection of both COVID-19 and influenza, the Flowflex Plus COVID-19 and Flu A/B Home Test plays an important role in differentiating between these illnesses, which present with similar symptoms but require potentially different response and treatments.
The Flowflex Plus COVID-19 and Flu A/B Antigen Home Test is designed to offer fast and accurate results within 15 minutes and can be performed at home, making it convenient for widespread use.
The ease of use and rapid results make the Flowflex Plus Combo Test ideal for workplace, point-of-care, and home settings.
DrugTestKitUSA remains committed to delivering cutting-edge diagnostic tools that meet the evolving challenges of the healthcare industry. The introduction of the Flowflex Plus COVID-19 and Flu A/B Home Test underscores this commitment by providing a reliable, easy-to-use solution for detecting both COVID-19 and influenza, empowers the consumer to participate in their healthcare.
For more information about the Flowflex Plus Combo Test and DrugTestKitUSA’s comprehensive range of diagnostic products, please visit DrugtestkitUSA.com

Drug use in the workplace is a real issue that many small to medium business (SMB) owners face. According to National Survey on Drug Use

The AvertD test is a prescription-only genetic test for those 18+ to assess the risk of opioid use disorder using a cheek swab.

Security at Stripe: Learn how Stripe handles security.
A PCI-certified auditor has audited Stripe. We’re a certified PCI Service Provider Level 1. This is the most stringent level of certification available in the payments industry. To accomplish this, we use the best-in-class security tools and practices to maintain a high level of security at Stripe.
Stripe forces HTTPS for all services using TLS (SSL), including our public website and the Dashboard to ensure secure connections:
We regularly audit the details of our implementation, including the certificates we serve, the certificate authorities we use, and the ciphers we support. We use HSTS to ensure that browsers interact with Stripe only over HTTPS. Stripe is also on the HSTS preloaded lists for both Google Chrome and Mozilla Firefox.
All card numbers are encrypted at rest with AES-256. Decryption keys are stored on separate machines. None of Stripe’s internal servers and daemons can obtain plain text card numbers but can request that cards are sent to a service provider on a static allowlist. Stripe’s infrastructure for storing, decrypting, and transmitting card numbers runs in a separate hosting environment, and doesn’t share any credentials with Stripe’s primary services including our API and website.
Why use PayPal?
Your information is secured.
When you pay with PayPal, your financial information is never shared with the seller, and you can pay using only your email address and password.
Security around the clock.
PayPal monitors every transaction 24/7 to help prevent against fraud, email phishing and identity theft. Every transaction is heavily guarded behind their next-level encryption.
If something seems suspicious, their dedicated team of security specialists is immediately on it to help protect you from fraudulent transactions. And remember, PayPal will never ask for any sensitive information from you in an email.
You can be sure that your transaction is protected and secure when you use PayPal at the DrugTestKitUSA checkout.