
How Long Does Cocaine Stay in Urine, Blood, Saliva, and Hair?
Cocaine is one of the most commonly screened substances in workplace and clinical drug testing. Whether testing is performed for employment, medical evaluation, or personal

Change in MAT for Opiate Abuse Regulations: Buprenorphine Prescribing
In 2021, nearly 107,000 people died of a drug overdose, with 75% of those deaths involving an opioid. Fentanyl was found to be the most common of these opioid-related deaths. However, buprenorphine was among the drugs used to reduce opioid drug abuse and fatalities associated with drug abuse. Due to its uses in reducing opioid misuse, and recent studies on the safety of buprenorphine in Medication Assisted Treatment of opioid abuse, the Drug Enforcement Administration (DEA) has now allowed practitioners to prescribe buprenorphine without prior notification via Notice of Intent. All practitioners who have a current DEA registration that includes Schedule III authority may now prescribe buprenorphine for Opioid Use Disorder in their practice.
The regulations for Medication-Assisted Treatment (MAT) for opiate abuse have undergone several changes in recent years. Here are some of the fundamental changes:
According to a recent study published in JAMA and also quoted by the National Institute of Health, researchers discovered that between July 2019 and June 2021, buprenorphine was only a factor in a very small number of drug overdose deaths. 1955 overdose deaths involving buprenorphine occurred during the research period, accounting for 2.2% of the overall drug overdose deaths and 2.6% of the opioid-related overdose deaths noted in the State Unintentional Drug Overdose Reporting System (SUDORS) dataset. The scientists found that although monthly opioid-related overdose deaths generally increased between April 2020 and June 2021 when DEA eased buprenorphine prescribing regulations in response to the COVID-19 pandemic, the proportion of those deaths involving buprenorphine did not increase.
The study also discovered that, in contrast to 67.2% of deaths using an opioid other than buprenorphine, 92.7% of overdose deaths involving buprenorphine also involved at least one other substance. Specifically, benzodiazepines, antidepressants, and anticonvulsants were more likely to be involved in buprenorphine-involved overdose deaths than other opioid-involved overdose deaths.
“Research has shown beyond a doubt that medications for opioid use disorder are overwhelmingly beneficial and can be lifesaving, yet they continue to be vastly underused,” said NIDA Director and senior author, Nora Volkow, M.D. “Expanding more equitable access to these medications for people with substance use disorders is a critical part of our nation’s response to the overdose crisis. The findings from this study strengthen existing evidence suggesting that greater flexibility in prescribing may be one safe method for working toward this goal.” quoted from the National Institute of Health
Here are some key points to consider regarding the recent change in policy around buprenorphine prescribing:
The recent removal of the federal requirement for practitioners to submit a Notice of Intent to prescribe medications like buprenorphine for treating Opioid Use Disorder is undoubtedly a positive step in expanding access to life-saving medications. However, this also raises some concerns about the potential for increased abuse of buprenorphine.
Buprenorphine is a partial opioid agonist that binds to the same receptors in the brain as opioids. It thereby reduces cravings and withdrawal symptoms. While it is an effective medication for treating OUD, it can also be used illicitly. Most illegal use of this drug, however, is for reducing withdrawal symptoms, decreasing amount of heroin or opiate use, and in some cases the financial cost of drug use.
As buprenorphine prescribed use, and in some cases, illicit use increases, the addition of buprenorphine to drug panels is beneficial. Buprenorphine drug tests are often used to ensure that the patient is taking their prescribed buprenorphine dose for treatment of opiate addiction. In the limited cases where it is being abused, buprenorphine can also be detected by a drug test.
DrugTestKitUSA offers a range of high-quality drug testing kits that are easy to use, accurate, and affordable. These drug testing kits can be an excellent tool for identifying potential substance use disorders. Many expanded panels are also capable of identifying buprenorphine in the system. Take advantage of the filtering feature to select from a variety of drug test attributes, including a filter for buprenorphine.

Cocaine is one of the most commonly screened substances in workplace and clinical drug testing. Whether testing is performed for employment, medical evaluation, or personal
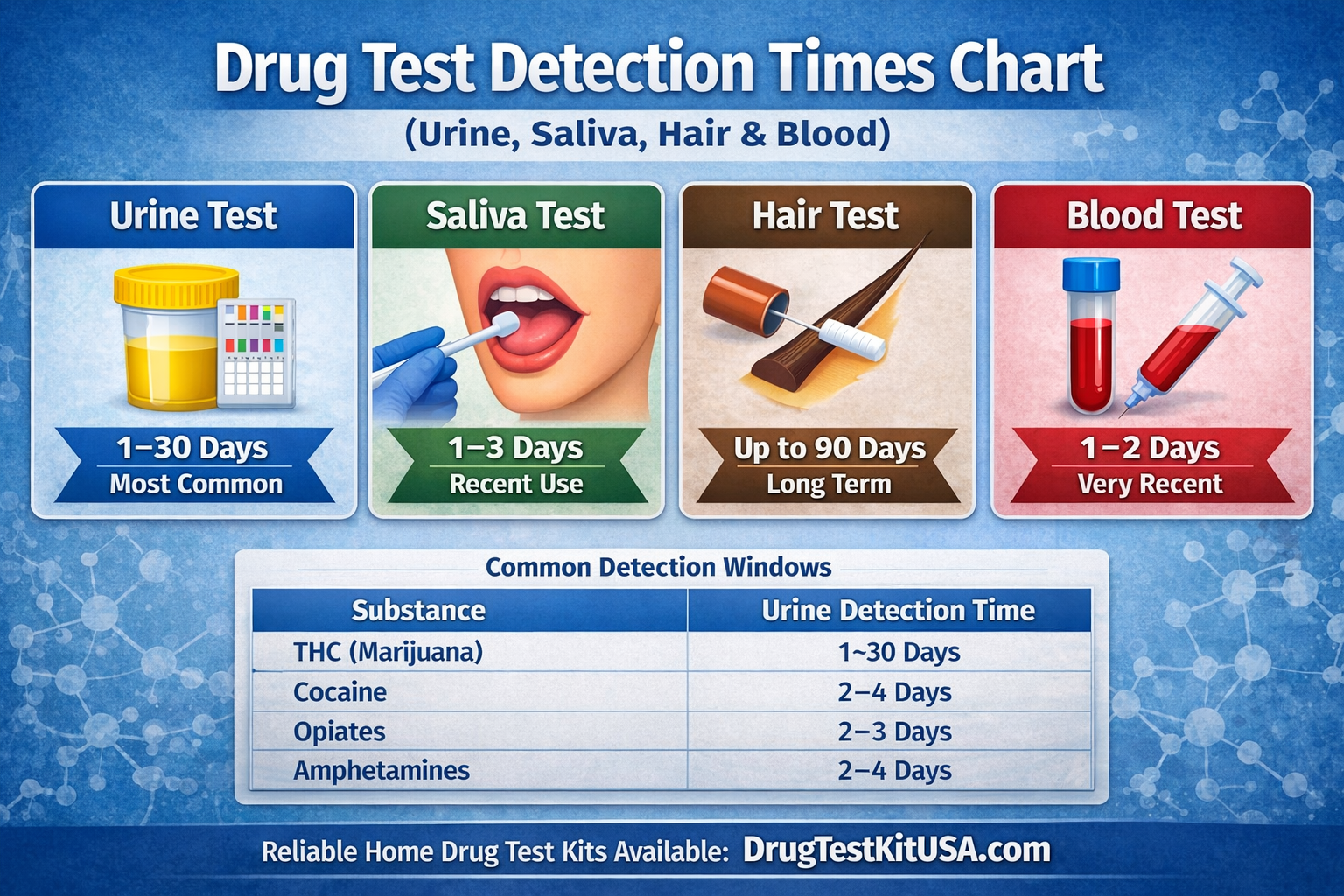
Drug test detection times vary depending on the substance, the type of test used, and individual biological factors. Understanding how long drugs remain detectable is

Security at Stripe: Learn how Stripe handles security.
A PCI-certified auditor has audited Stripe. We’re a certified PCI Service Provider Level 1. This is the most stringent level of certification available in the payments industry. To accomplish this, we use the best-in-class security tools and practices to maintain a high level of security at Stripe.
Stripe forces HTTPS for all services using TLS (SSL), including our public website and the Dashboard to ensure secure connections:
We regularly audit the details of our implementation, including the certificates we serve, the certificate authorities we use, and the ciphers we support. We use HSTS to ensure that browsers interact with Stripe only over HTTPS. Stripe is also on the HSTS preloaded lists for both Google Chrome and Mozilla Firefox.
All card numbers are encrypted at rest with AES-256. Decryption keys are stored on separate machines. None of Stripe’s internal servers and daemons can obtain plain text card numbers but can request that cards are sent to a service provider on a static allowlist. Stripe’s infrastructure for storing, decrypting, and transmitting card numbers runs in a separate hosting environment, and doesn’t share any credentials with Stripe’s primary services including our API and website.
Why use PayPal?
Your information is secured.
When you pay with PayPal, your financial information is never shared with the seller, and you can pay using only your email address and password.
Security around the clock.
PayPal monitors every transaction 24/7 to help prevent against fraud, email phishing and identity theft. Every transaction is heavily guarded behind their next-level encryption.
If something seems suspicious, their dedicated team of security specialists is immediately on it to help protect you from fraudulent transactions. And remember, PayPal will never ask for any sensitive information from you in an email.
You can be sure that your transaction is protected and secure when you use PayPal at the DrugTestKitUSA checkout.